Rapid Diagnostics at the Point of Care
Biosensors + Exhaled Breath Condensate Deliver Accurate Panel Testing
DiagMetrics’ revolutionary breath-based diagnostic test harnesses the power of exhaled breath condensate (EBC) to provide a non-invasive, efficient, and highly accurate method for detecting active infections and viral load.
The mask-based design simplifies the process, producing rapid results without compromising on accuracy. It offers a non-invasive, comfortable, and user-friendly experience, making it ideal for mass testing in a variety of settings, from laboratories to bedside to large population testing in municipal and public settings.
How It Works
This mask-based COVID-19 testing system uses a unique Exhaled Breath Collector (EBC) that converts breath to a liquid bio sample (condensate) for rapid testing and wireless automatic contact tracing.
1.
Sample Collection
The Exhaled Breath Condensate is collected from a fan-shaped condenser embedded in a mask, which then funnels the sample to our proprietary electronic biosensor.
2.
The Biosensor is Functionalized
The biosensor is functionalized using engineered COVID-19 antibody fragments as the capture molecules. These engineered antibody fragments have a strong and selective affinity for the COVID-19 S-protein. Once the S-protein attaches to the antibody fragments, the electrical characteristics of the biosensor change, resulting in a change in current flowing through the biosensor.
3.
Results in <10 Minutes
The change in current triggers a signal that is sent via Bluetooth to a smartphone app, which then signals to the user a “positive” test for COVID-19. This smartphone app can then be used to perform contact tracing. If the change in current is below a predetermined threshold, the test will be deemed “negative.” When a test is negative, the full testing system can be reused. Our electronic biosensor has been proven to detect low concentration levels of the COVID-19 S-protein and typically takes less than 10 minutes for a test to be completed.
Molecular Diagnostics
DiagMetrics’ revolutionary breath-based diagnostic test harnesses the power of exhaled breath condensate (EBC) to provide a non-invasive, efficient, and highly accurate method for detecting active infections and viral load.
Non-Invasive Sampling
DiagMetrics’ point-of-care test employs a non-invasive method to collect Airway Lining Fluid (ALF) through EBC. This eliminates the need for uncomfortable and invasive procedures.
Rich Biomarker Analysis
The patent-pending technology can analyze over 2,000 distinct biomarkers within EBC, enabling precise and comprehensive diagnostic insights.
Cleaner Bio-Sample
EBC is a cleaner bio-sample compared to alternatives like saliva and mucous. It contains over 99% water and lacks confounding molecules such as salts and stray proteins, ensuring the accuracy of test results.
Ease of Collection
Obtaining an EBC sample is easy and requires no medical intervention. Simply breathing into the mask makes it accessible for a wide range of individuals.
RADx® NIH Funding Accelerates Development
DiagMetrics has been awarded Phase 1 funding from the National Institutes of Health (NIH) Rapid Acceleration of Diagnostics (RADx®) Tech program.
COVID-19 Diagnostics
- Demonstrated functionality of all components
- Biosensor response to COVID-19 virus is consistent, have detected all variants to date
- Masks can be used as a bio-sample collection system for research
- Extend biosensor to detect Flu A/Flu B/RSV in addition to COVID-19
- Extend the system for people with accessibility needs
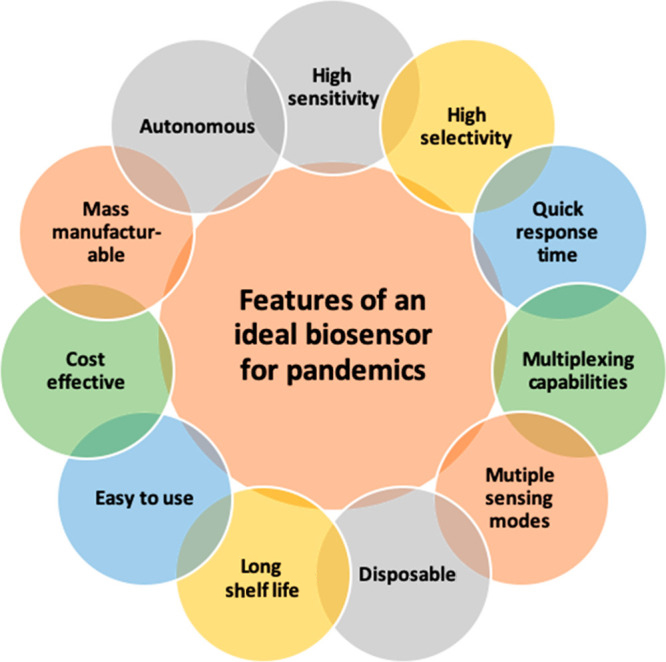
Image courtesy of Opportunities and Challenges for Biosensors and Nanoscale Analytical Tools for Pandemics: COVID-19, Bhalla et al., ACS Nano 2020 14 (7), 7783-7807, DOI: 10.1021/acsnano.0c04421
Semiconductor-Based Biosensor
Accessible, Affordable COVID-19 Tests at Home or In the Field
DiagMetrics’ electrochemical semiconductor biosensor detects target molecules, like the N- proteins of the SARS-CoV-2 virus, making it the ideal pandemic test system.
The biosensor sends a direct-to-electrical signal, wirelessly transmitting test results in minutes.
IP Portfolio
DiagMetrics’ aggressive IP protection strategy aims at building a world-class U.S. and International Patent Portfolio.
- 7 US patents pending
- 4 patent applications cover multiple features and alternative constructions of mask-based EBC collection, biosensors, methods of manufacturing and specific disease use-cases
- First PCT patent converted to national filings in Asia, EU including UK, North and South America
- International search shows strong patentability for key features
- Multiple patents pending on diagnosis of diseases with biomarkers in EBC

Product Roadmap
Our technology and its enhancements and derivatives are a platform for near term follow-on use-cases.
Pandemics / Endemics Diagnostics
- SARS/Flu A/Flu B/RSV – our technology is ready for simultaneous multiple biomarker panel tests
Tuberculosis
- The CDC estimates 23% of the global population are currently infected with TB
- TB is increasingly becoming drug-resistant and affecting other organs in the body and is one of the most infectious diseases
Cardiac Disease Detection, Remote Patient Monitoring
- Daily and periodic monitoring
- Cardiac diseases are the leading cause of death worldwide
- More than 20 million fatalities annually, over 586 million annual cases, up >27% from 2010
Difficult/late to diagnose conditions with very high mortality rates
- Lung cancer has biomarkers in exhaled breath for early detection